- Your cart is empty
- Continue Shopping

Shop
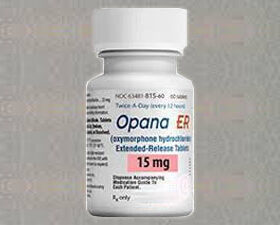
Buy Opana ER Online

Opana ER from a trusted source, delivered right to your doorstep in record time. We offer a convenient and hassle-free way to obtain your medication quickly and discreetly. Trust us for all your medication needs – we’ve got you covered.
Introduction
- Opana ER (extended-release oxymorphone) is a prescription opioid pain medication that was first approved by the FDA in 2006. It is an extended-release formulation of oxymorphone, a semi-synthetic opioid analgesic.
- Opana ER was developed and manufactured by Endo Pharmaceuticals to treat severe chronic pain in patients requiring continuous, around-the-clock opioid treatment for an extended period of time. It is intended for use in opioid-tolerant patients only.
- Opana ER belongs to the broader class of prescription opioid pain relievers. These medications work by binding to opioid receptors in the brain and spinal cord to reduce the perception of pain. While effective for treating pain, opioids like Opana ER also carry a high risk of abuse and addiction.
Abuse Potential
Opana ER has been associated with higher rates of abuse compared to other prescription opioid painkillers. The original formulation of Opana ER had a much higher potential for abuse than the reformulated version released in 2012.
Several factors contributed to the high abuse rates of original Opana ER:
- It could be crushed or dissolved, making it easy to snort or inject. This allowed users to bypass the extended-release mechanism and get the full dose all at once.
- The active ingredient oxymorphone is more potent than other opioid ingredients like oxycodone. It produces stronger euphoric effects when taken in large doses.
- The extended-release effect made the pills last longer, giving a more prolonged high compared to immediate-release opioids.
- When crushed, Opana ER released the oxymorphone faster than other extended release opioids like OxyContin. This made it more attractive to opioid abusers.
- The coating on the pills was easy to remove, making the pills simple to manipulate and abuse through snorting or injection.
Because of these factors, the rates of abuse, addiction, overdose, and injection-related disease like HIV were much higher for Opana ER than comparable long-acting opioids. This led the FDA to eventually request it be reformulated to be more abuse-deterrent.
Reformulation
In 2017, Opana ER was reformulated by Endo Pharmaceuticals in an attempt to make the drug more difficult to manipulate and abuse. The original formulation of Opana ER had a higher potential for abuse because it could be crushed into powder and then snorted or injected.
The new formulation uses inactive ingredients that make the pills harder to crush. When crushed, the reformulated Opana ER becomes viscous and more difficult to snort or inject.
Endo spent over $100 million developing the abuse-deterrent formulation of Opana ER. They claimed this would help prevent the misuse and abuse of the powerful opioid painkiller.
Impacts of Withdrawal
The withdrawal of Opana ER from the market in 2017 had significant impacts on patients, healthcare providers, and the pharmaceutical company Endo.
- For patients taking Opana ER, the sudden removal of the drug from pharmacies was highly disruptive. Many patients reported withdrawal symptoms and difficulties finding alternative treatments. Doctors were forced to rapidly transition patients to different medications, which was burdensome for providers and patients alike.
- However, the withdrawal also had benefits. Removing Opana ER helped curb the alarming rates of abuse and misuse. While alternative opioids carry risks too, the reformulated Opana ER was clearly prone to injection and contributing to viral outbreaks. Eliminating this version from the market may have prevented further abuse.
- Overall, the withdrawal delivered a mix of drawbacks and benefits for those involved. Patients and doctors struggled with the sudden transition, but it may have prevented further public health damages. The company lost substantial revenue, but also the liabilities of an abused drug. There were reasonable rationales on both sides of the withdrawal decision.
Alternatives
- Opana ER was an extended-release oxymorphone product that was widely prescribed for chronic moderate to severe pain. After its withdrawal from the market in 2017, patients and healthcare providers had to transition to alternative treatment options.
- Other opioid medications are still available to help manage chronic pain, including other long-acting formulations. Oxycodone ER (OxyContin) is probably the most well-known, along with morphine ER, hydromorphone ER, fentanyl patches, and methadone. These can provide similar around-the-clock analgesia like Opana ER.
- The main drawback with these alternatives is they are all still opioids. So while they may effectively treat pain, they have the same addiction, dependence, and abuse issues inherent to this class of medications. This highlights the need for a multifaceted approach to chronic pain management, including non-opioid medications, physical therapy, behavioral techniques, and lifestyle changes.
Conclusion
Opana ER illustrates the complex issues surrounding prescription opioid abuse and reformulation. Initially approved as a long-acting opioid analgesic for severe chronic pain, Opana ER gained notoriety for its high potential for abuse and misuse. The original formulation led to crushing and snorting of pills to override the extended-release mechanism and produce a heroin-like high.
The complex issues highlighted by Opana ER – balancing pain relief with abuse potential, unintended consequences of reformulation, role of manufacturers and regulators – reflect broader debates regarding the prescription opioid epidemic. More responsible prescribing practices, abuse-deterrent formulations, prevention education, treatment access, and other measures are needed to address this public health crisis. The story of Opana ER provides insights into the challenges ahead.